Life Sciences, in this case, the pharmaceutical and medical device sectors, have various applications in which 3D printing can contribute real value. The needs are very specific and there are a few crucial requirements:
In clinical applications, all of these requirements hold true. But what’s more is that the printer must be safe to use and parts must be able to be sterilized. For medical devices, parts must also be very strong so they can be used in functional settings.
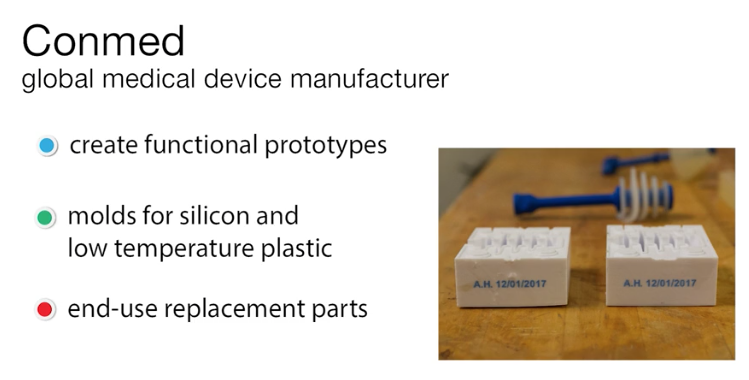
The Rize One provides a safe and secure solution the Life Sciences. The patented ADP process creates near isotropic parts, with minimal post processing and zero Volatile Organic Compounds or VOCs. In this case study Conmed, a global medical device manufacturer, had a need for parts with great surface quality, strength and markup.
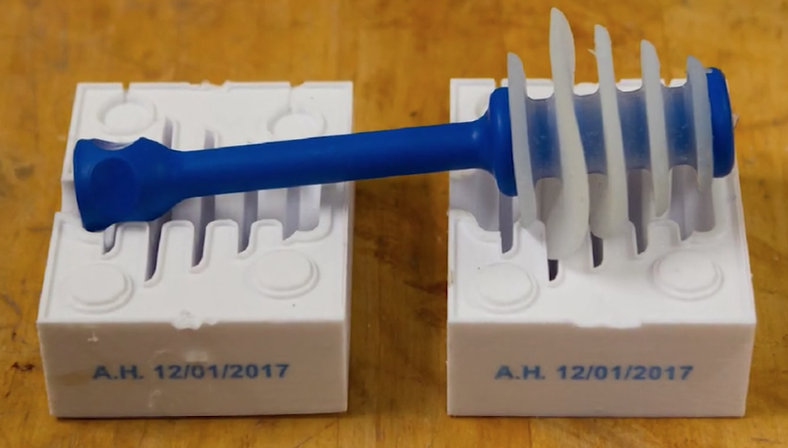
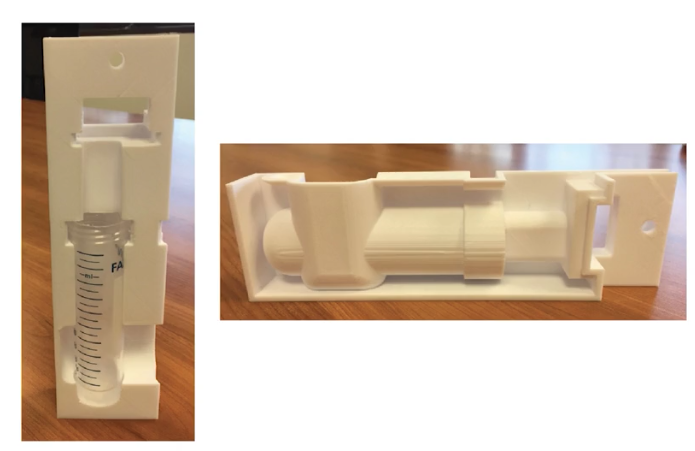
Using Rize they were able to first create functional prototypes, followed by molds for pouring silicon and low-temperature plastic. After realizing the possibilities, they expanded their use of the Rize to create end-use replacement parts: handles, other parts of assemblies, and tooling. An added benefit of the Rize is in its ability of jetting marking inks, which allows Conmed to efficiently trace parts in their inventory.
Aside from Medical Devices, other areas where the combination of the APD process and a material well suited for the task includes; pharmaceutical pre-clinical and clinical applications. While Rizium one is not yet USP Class VI certified, all of its components are which means it should be soon and supports its claims about material safety.
As you can see, the Rize One is a safe and environmentally-friendly solution that can enhance productivity in the medical field and ultimately cut costs. Please be sure to sign up for our 2 Minute Tuesday video series to receive tips and tricks like this one in video form every week. More info at the button below.








Leave A Comment